Virus Capture
The size of viruses makes them difficult filter out from the water supply or capture in sensors. For example, filter pore sizes below 200 nm drastically reduce fluid flow and most virus are substantially smaller than this. Common viruses such as Influenza (100 nm), Rotavirus and the Coronaviruses (~100nm) are all smaller than is practical to remove by mechanical size exclusion alone. In some applications such as in the blood, one wants large bodies such as red blood cells (10 cells) to pass through whilst simultaneously catching small viruses such as Hepatitis C (~50nm).
For these reasons, when targeting moieties below one micron, electrokinetic/electrostatic approaches are often exploited. These rely on the zeta potential of nanoparticles in solution, i.e. the net charge particles exhibit when their surface functional groups react with solutions. For the majority of viruses, this charge is negative over the physiological pH range. Unfortunately, this is also true for the overwhelming majority of filter materials such as polymers, cellulose, glass quartz etc. Thus, they repel each other and viruses are able to pass through small pores in filters with ease.
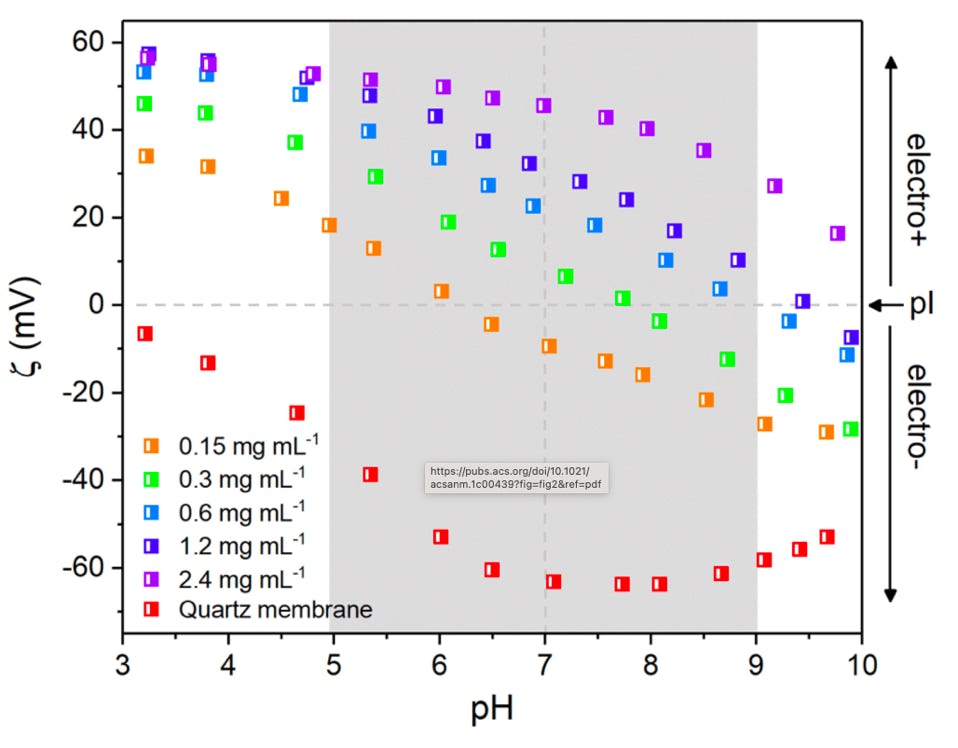
Diamond nanoparticles can be made to exhibit an anomalously strong positive zeta potential across the entire pH range (see nanodiamond). We have shown by electrostatically assembling these particles onto conventional filter membranes, we are able to reverse the filter zeta potential from negative to positive charge across a wide pH range. The graph left shows that quartz membranes are negatively charged from pH 3 to 10. By exposing the filter to increasing concentrations (from 0.15 to to 2.4 mg/mL) of diamond nanoparticles in solution, the zeta potential becomes increasingly positive until it exhibits a strong positive zeta potential across the entire pH range. The grey area represents the pH range within the body.
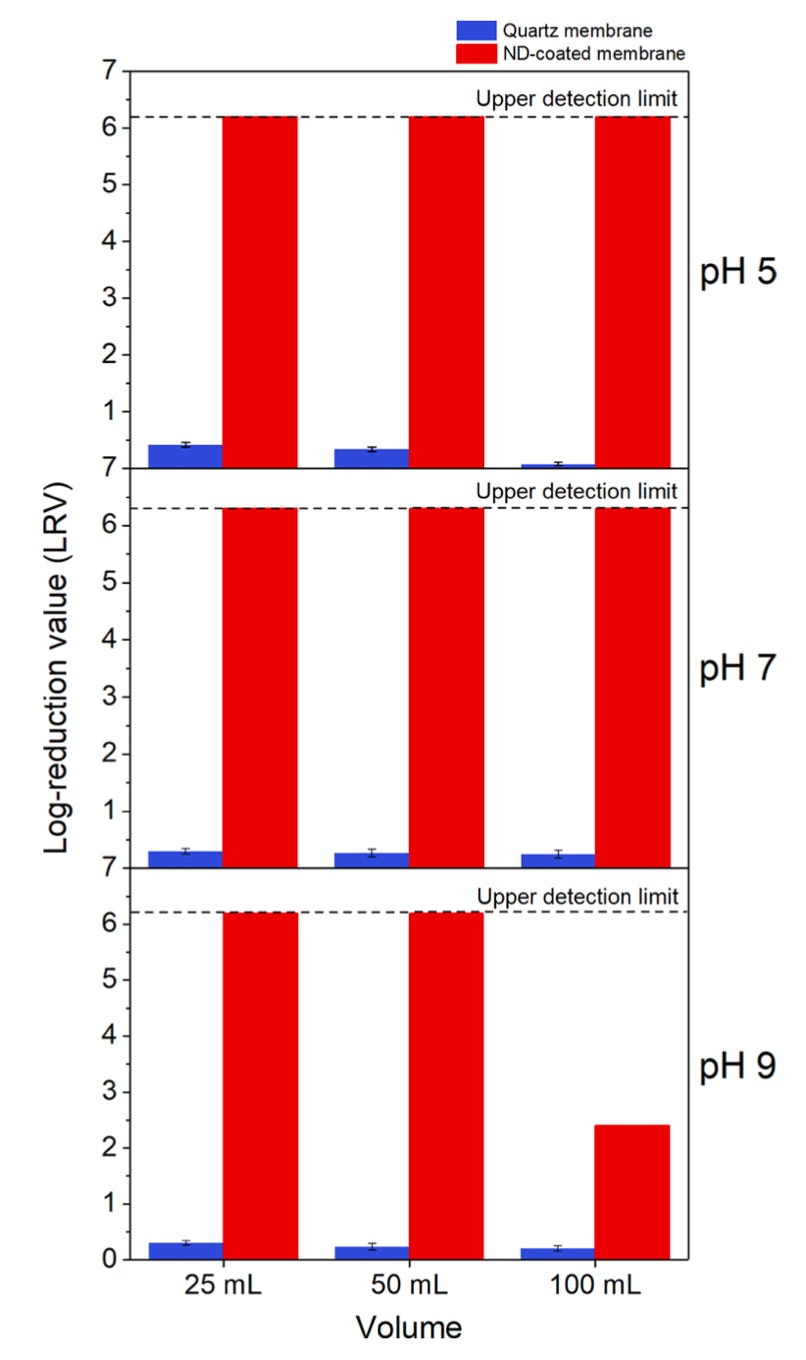
The nanodiamond modified filters have a strong affinity for viruses. For example, the bacteriophage MS2 was used to test the efficacy of the filter (see histograms right). An untreated filter (blue bars) has almost no capability to remove viruses, which is not surprising given that the pores are several micron (see electron micrograph below) and the MS2 virus <30nm. The treated filter removes log6 (>99.9999%) of the MS2 virus, with a slight reduction in efficacy at pH 9. This is due to the weaker zeta potential at this pH even though it is significantly stronger than commercially available positive charged membranes. See:
HA Bland et al, ACS Applied Nano Materials, 4 (2021) 3252

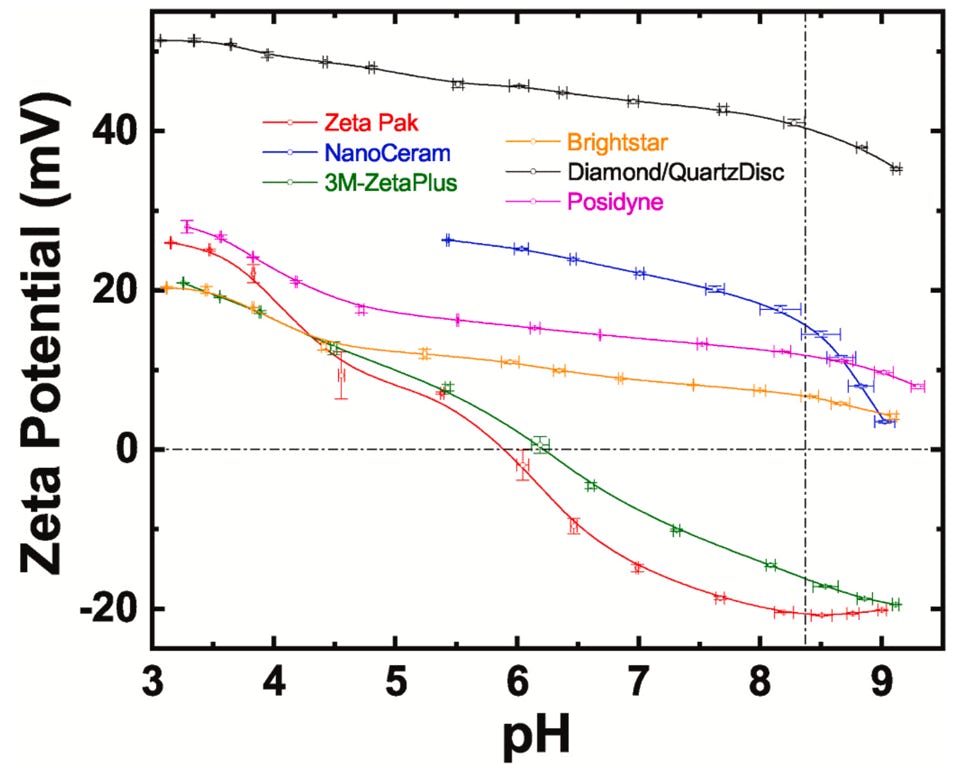
Recently, we have benchmarked our electropositive filter technology against commercially available electropositive filters. The graph right shows the variation of zeta potential with pH for the various filters. It is clear that the diamond nanoparticle enhanced filters exhibit a higher positive zeta potential across a wider pH range than those available commercially. A more complete discussion is available here: